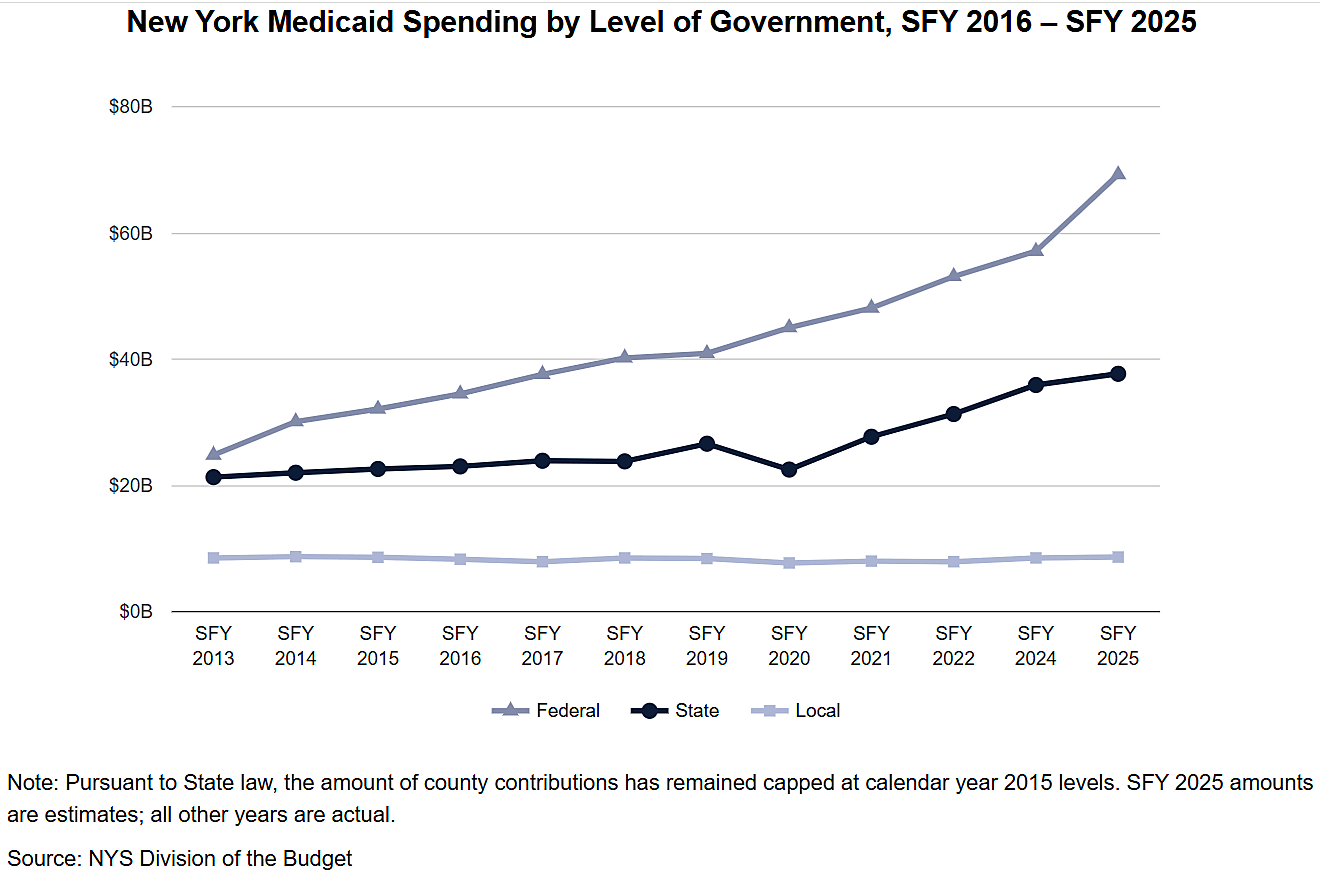
On January 29, President Trump signed an executive order called The Great American Recovery Initiative. The order does not significantly reform addiction policy or change how treatment is provided. Instead, it creates a White House–level coordination and messaging effort to align federal programs, guide grant-making, and promote a specific narrative about addiction and recovery. In practice, it supports the same enforcement- and treatment-focused framework already in place, while keeping the regulatory structures that limit access, restrict choices, and make effective, low-barrier recovery more difficult.
The Initiative is co-chaired by the Secretary of Health and Human Services and a Senior Advisor for Addiction Recovery, who is not yet appointed. It includes an executive director, cabinet officials, and senior administration leaders, some of whom have personal experience with addiction.
Unfortunately, the initiative rests heavily on the claim that addiction is a “chronic disease,” a framing that obscures more than it clarifies. Addiction medicine specialists typically characterize addiction as compulsive behavior despite negative consequences—a behavioral pattern shaped by learning, incentives, and environment rather than a progressive biological disease akin to diabetes or hypertension.
Many scholars, such as Maia Szalavitz, have argued that addiction is better understood as a learning or behavioral disorder, often rooted in early developmental trauma and frequently intertwined with neuropsychiatric comorbidities such as autism spectrum disorder, obsessive-compulsive disorder, ADHD, and PTSD. Addictive behavior isn’t limited to substances. For example, gambling addiction and sex addiction are recognized types of addiction disorders.
Treating addiction primarily as a disease risks reinforcing centralized, medicalized, and clinic-bound models of care, while leaving untouched the federal policies that restrict access, limit consumer choice, and make recovery harder than it needs to be.
As shown in the award-winning film Shuffle, which we screened at the Cato Institute last December, many clinic-bound models exploit the mandates and regulatory framework set by the Affordable Care Act to siphon off millions of dollars from insurance companies, recycling patients into revenue streams and harming them in the process.
Another example of the centralized care model, which stems from a misunderstanding of addiction, is the requirement that individuals can only receive methadone treatment for opioid dependency and addiction—proven, for decades, to be the most effective form of treatment—at Drug Enforcement Administration (DEA)-approved clinics called opioid treatment programs (OTPs), where they must line up every day and take the medication in front of staff.
Readers are justified if they are confused that a law enforcement agency has the authority to decide where, how, and from whom people with opioid use disorder (OUD) can receive treatment. Unfortunately, unlike in Australia, Canada, and the UK, cops practice medicine in the US. In those other countries, patients have been obtaining methadone through their primary care providers in collaboration with pharmacists for over fifty years. American patients accessed methadone from their health care providers before 1972. Then, the Controlled Substances Act ended that practice and segregated patients with addiction into DEA-approved clinics.
Because of the punitive OTP system, only about 600,000 people with OUD received methadone in 2024, though 8 million residents over age 12 met criteria for OUD that year. A 2019 report from the Congressional Research Service found that 80 percent of US counties had no OTPs. Wyoming has no OTPs. People with OUD have to travel to another state to get their daily, in-person dose of methadone, which is not practical.
Legal scholars at the American Society for Addiction Medicine (ASAM) have argued, without success, that the executive branch can address the problem with regulatory reform.
If the Trump administration wants a great American recovery initiative, it should ask Congress to expand access to methadone treatment by removing barriers to primary care clinicians prescribing methadone, as Sofia Hamilton and I explained in a 2023 policy analysis. Expanding access would also shrink the market for unscrupulous, predatory rehab clinics.
A bipartisan group of lawmakers in both houses of Congress sponsored the Modernizing Opioid Treatment Access Act (MOTAA) in 2023. It would have enabled patients to access methadone from board-certified addiction specialists alongside OTPs. Although it didn’t go nearly far enough—there are not enough board-certified specialists to meet demand, and pilot programs in the US show primary care clinicians can competently prescribe methadone treatment—it was a step in the right direction. The bill did not advance, and lawmakers in the current Congress have not reintroduced it.
Real recovery will not come from another White House initiative or a better-crafted narrative about addiction. It will come from treating people with addiction like everyone else—capable of making choices, able to obtain care without special permissions or carve-outs, and not in need of a law-enforcement–run treatment system. Until federal policy reflects that basic premise, “recovery” will remain more of a slogan than a solution.